 | |
| Names | |
|---|---|
| Other names
1,1'-trithiaferrocenophane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| |
| Properties | |
| C10H8FeS3 | |
| Molar mass | 280.20 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.887 g/cm3[1] |
| Melting point | 149.5–150.5 °C (301.1–302.9 °F; 422.6–423.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
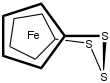
1,1'-Ferrocenetrisulfide is the organoiron compound with the formula Fe(C5H4S)2S. A yellow solid, it is the simplest polysulfide derivative of ferrocene. It can be synthesized by treatment of dilithioferrocene with elemental sulfur.[2] Using proton NMR spectroscopy, the relatively slow conformational flexing of the trisulfide ring can be established.
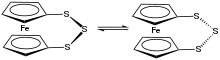 Conformational equilibrium for ferrocene-1,1'-trisulfide.
Conformational equilibrium for ferrocene-1,1'-trisulfide.
References
- ↑ Davis, Betty R.; Bernal, Ivan (1972). "Structure of a novel fluxional molecule: 1,2,3-trithia-[3]-ferrocenophane". Journal of Crystal and Molecular Structure. 2 (3): 107–114. doi:10.1007/BF01464791. S2CID 96187454.
- ↑ Bishop, J.J.; Davison, A.; Katcher, M.L.; Lichtenberg, D.W.; Merrill, R.E.; Smart, J.C. (1971). "Symmetrically disubstituted ferrocenes". Journal of Organometallic Chemistry. 27 (2): 241–249. doi:10.1016/S0022-328X(00)80571-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.