 | |
| Names | |
|---|---|
| IUPAC name
Gold(III) oxide | |
| Other names
Gold trioxide, Gold sesquioxide, Auric oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.013.748 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Au2O3 | |
| Molar mass | 441.93 |
| Appearance | red-brown solid |
| Density | 11.34 g/cm3 at 20 °C[1] |
| Melting point | 298 °C (568 °F; 571 K)[2] |
| insoluble in water, soluble in hydrochloric and nitric acid | |
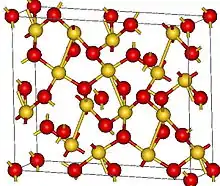
| Structure | |
| Orthorhombic, oF40 | |
| Fdd2, No. 43[1] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gold(III) oxide (Au2O3) is an inorganic compound of gold and oxygen with the formula Au2O3. It is a red-brown solid that decomposes at 298 °C.[3]
According to X-ray crystallography, Au2O3 features square planar gold centers with both 2- and 3-coordinated oxides. The four Au-O bond distances range from 193 to 207 picometers.[1] The crystals can be prepared by heating amorphous hydrated gold(III) oxide with perchloric acid and an alkali metal perchlorate in a sealed quartz tube at a temperature of around 250 °C and a pressure of around 30 MPa.[4]
References
- 1 2 3 Jones, P. G.; Rumpel, H.; Schwarzmann, E.; Sheldrick, G. M.; Paulus, H. (1979). "Gold(III) oxide". Acta Crystallographica Section B. 35 (6): 1435. doi:10.1107/S0567740879006622.
- ↑ Kawamoto, Daisuke; Ando, Hiroaki; Ohashi, Hironori; Kobayashi, Yasuhiro; Honma, Tetsuo; Ishida, Tamao; Tokunaga, Makoto; Okaue, Yoshihiro; Utsunomiya, Satoshi; Yokoyama, Takushi (2016-11-15). "Structure of a Gold(III) Hydroxide and Determination of Its Solubility". Bulletin of the Chemical Society of Japan. The Chemical Society of Japan. 89 (11): 1385–1390. doi:10.1246/bcsj.20160228. ISSN 0009-2673.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Jones, Peter G.; Rumpel, Horst; Sheldrick, George M.; Schwarzmann, Einhard (1980). "Gold(III) oxide and oxychloride" (open access). Gold Bulletin. 13 (2): 56. doi:10.1007/BF03215453.
External links
 Media related to Gold(III) oxide at Wikimedia Commons
Media related to Gold(III) oxide at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
