 Pr3+ O2− | |
-oxide.jpg.webp) | |
| Names | |
|---|---|
| IUPAC name
Praseodymium(III) oxide | |
| Other names
Praseodymium oxide, Praseodymium sesquioxide | |
| Identifiers | |
| ECHA InfoCard | 100.031.665 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| Pr2O3 | |
| Molar mass | 329.813 g/mol |
| Appearance | light green solid |
| Density | 6.9 g/cm3 |
| Melting point | 2,183 °C (3,961 °F; 2,456 K) |
| Boiling point | 3,760 °C (6,800 °F; 4,030 K)[1] |
| +8994.0·10−6 cm3/mol | |
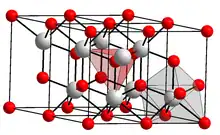
| Structure | |
| Hexagonal, hP5 | |
| P-3m1, No. 164 | |
| Thermochemistry | |
Heat capacity (C) |
117.4 J•mol−1•K−1[1] |
Std enthalpy of formation (ΔfH⦵298) |
-1809.6 kJ•mol−1 |
| Related compounds | |
Other anions |
Praseodymium(III) chloride Praseodymium(III) sulfide |
Other cations |
Neodymium(III) oxide Promethium(III) oxide Cerium(III) oxide |
Related compounds |
Uranium(VI) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Praseodymium(III) oxide, praseodymium oxide or praseodymia is the chemical compound composed of praseodymium and oxygen with the formula Pr2O3. It forms light green hexagonal crystals.[1] Praseodymium(III) oxide crystallizes in the manganese(III) oxide or bixbyite structure.[2]
Uses
Praseodymium(III) oxide can be used as a dielectric in combination with silicon.[2] Praseodymium-doped glass, called didymium glass, turns yellow and is used in welding goggles because it blocks infrared radiation. Praseodymium(III) oxide is also used to color glass and ceramics yellow.[3] For coloring ceramics, also the very dark brown mixed-valence compound praseodymium(III,IV) oxide, Pr6O11, is used.
References
- 1 2 3 Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 478, 523, ISBN 0-8493-0594-2
- 1 2 Dabrowski, Jarek; Weber, Eicke R. (2004), Predictive Simulation of Semiconductor Processing, Springer, p. 264, ISBN 978-3-540-20481-7, retrieved 2009-03-18
- ↑ Krebs, Robert E. (2006), The History and Use of our Earth's Chemical Elements, Greenwood Publishing Group, p. 283, ISBN 978-0-313-33438-2, retrieved 2009-03-18
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.