 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dibromopropane[1] | |
| Other names
Propylene dibromide[2] | |
| Identifiers | |
3D model (JSmol) |
|
| 1718884 | |
| ChemSpider | |
| ECHA InfoCard | 100.001.036 |
| EC Number |
|
| MeSH | 1,2-dibromopropane |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6Br2 | |
| Molar mass | 201.889 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.937 g mL−1 |
| Melting point | −55.5 °C; −67.8 °F; 217.7 K |
| Boiling point | 139 to 143 °C; 282 to 289 °F; 412 to 416 K |
Henry's law constant (kH) |
6.7 μmol Pa−1 kg−1 |
Refractive index (nD) |
1.519 |
| Thermochemistry | |
Heat capacity (C) |
172.8 J K mol−1 |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H226, H302, H332 | |
| Flash point | 50 °C (122 °F; 323 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
741 mg kg−1 (oral, rat) |
| Related compounds | |
Related alkanes |
|
Related compounds |
Mitobronitol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
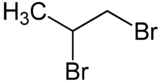
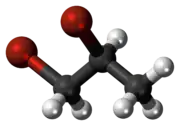
1,2-Dibromopropane, also known as propylene dibromide, is an organic compound with the formula CH3CHBrCH2Br. It is the simplest chiral hydrocarbon containing two bromine atoms:
.png.webp)
(S)-1,2-Dibrompropane (above) and (R)-1,2-Dibrompropane (below)
References
1,2-Dibromo Propane also known as Propylene bromide is a naturally occurring organic compound. It is part of the Vicinal Dihalide family; it is highly unstable due to both torsional strain and its highly electrophilic nature.
- ↑ "1,2-dibromopropane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification. Retrieved 21 June 2012.
- ↑ "PubChem". pubchem.ncbi.nlm.nih.gov.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.