 | |
| Names | |
|---|---|
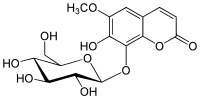
| IUPAC name
8-(β-D-Glucopyranosyloxy)-7-hydroxy-6-methoxy-2H-1-benzopyran-2-one | |
| Systematic IUPAC name
7-Hydroxy-6-methoxy-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2H-1-benzopyran-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.597 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H18O10 | |
| Molar mass | 370.310 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Fraxin is a glucoside of fraxetin.[1] Fraxin extracted from ash bark exhibits florescence in aqueous solution.[2] A blue/green luminescence can be observed by soaking ash twigs in hot water.
Bibliography
- ↑ Hirsch, Anne-Marie; Longeon, Arlette; Guyot, Michèle (January 2002). "Fraxin and esculin: two coumarins specific to Actinidia chinensis and A. deliciosa (kiwifruit)". Biochemical Systematics and Ecology. 30 (1): 55–60. doi:10.1016/S0305-1978(01)00064-3.
- ↑ Meikle, R. D. (1958). British Trees and Shrubs (Kew Series) (1st ed.). UK: Eyre & Spottiswoode. pp. 129–132.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.