 | |
| Names | |
|---|---|
| IUPAC name
Titanium(III) iodide | |
| Other names
Titanium triiodide | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| I3Ti | |
| Molar mass | 428.580 g·mol−1 |
| Appearance | black-violet solid |
| Density | 4.96 g·cm−3[1] |
| Related compounds | |
Other anions |
Titanium(III) fluoride Titanium(III) bromide Titanium(III) chloride |
Other cations |
Zirconium(III) iodide Hafnium(III) iodide |
Related compounds |
Titanium(IV) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Titanium(III) iodide is an inorganic compound with the formula TiI3. It is a dark violet solid that is insoluble in solvents, except upon decomposition.
Preparation and structure
Titanium(III) iodide can be prepared by reaction of titanium with iodine:[2]
It can also be obtained by reduction of TiI4, e.g., with aluminium.[3]
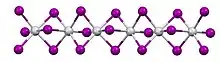
In terms of its structure, the compound exists as a polymer of face-sharing octahedra. Above 323 K, the Ti---Ti spacing are equal, but below that temperature, the material undergoes a phase transition. In the low temperature phase, the Ti---Ti contacts are alternating short and long. The low temperature structure is similar to that of molybdenum tribromide.[1]
References
- 1 2 Angelkort, Joachim; Schoenleber, Andreas; van Smaalen, Sander (2009). "Low- and High-Temperature Crystal Structures of TiI3". Journal of Solid State Chemistry. 182: 525–53. doi:10.1016/j.jssc.2008.11.028..
- ↑ F. Hein, S. Herzog "Molybdenum(III) Bromide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1407.
- ↑ Catherine E. Housecroft, A. G. Sharpe (2005), Inorganic Chemistry (in German), Pearson Education, p. 601, ISBN 0-13039913-2
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.