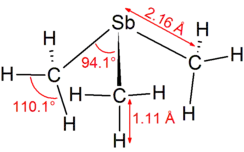 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Trimethylstibane[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.933 |
| EC Number |
|
| MeSH | trimethylantimony |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C 3SbH 9 | |
| Molar mass | 166.86 g mol−1 |
| Appearance | Colourless liquid |
| Density | 1.523 g cm−3 (at 15°C) |
| Melting point | −62 °C (−80 °F; 211 K) |
| Boiling point | 81 °C (178 °F; 354 K) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
24-26 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
-2.896--2.946 MJ mol−1 |
| Related compounds | |
Related compounds |
Trimethylamine Trimethylphosphine Trimethylarsine Triphenylstibine Trimethylbismuth |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Trimethylstibine is an organoantimony compound with the formula Sb(CH3)3. It is a colorless pyrophoric and toxic liquid.[2] It is synthesized by treatment of antimony trichloride and methyl Grignard reagent.[3] It is produced by anaerobic bacteria in antimony-rich soils.[4] In contrast to trimethylphosphine, trimethylstibine is a weaker Lewis base. It is used in the production of some III-V semiconductors.
References
- ↑ "trimethylantimony - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. 26 March 2005. Descriptors Computed from Structure. Retrieved 25 September 2011.
- ↑ Wiberg, Nils; Wiberg, Egon; Holleman, A. F. (2001), Inorganic Chemistry, Academic Press, p. 766, ISBN 0-12-352651-5, retrieved 2009-07-17
- ↑ Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf "Antimony and Antimony Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_055.pub2
- ↑ Craig, P. J. (2003), Organometallic Compounds in the Environment (2 ed.), Wiley and Sons, p. 295, ISBN 978-0-471-89993-8, retrieved 2009-07-17
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.